.avif)
2025 HSC Chemistry Exam Paper Exemplar Answers & Solutions

Can't wait for the solutions to the 2025 HSC Chemistry exam paper solutions to be released? Are you finding the publicly available solutions and sample answers inadequate?
Not to worry! The state-ranking tutors at Cognito have your back! 💪
Section I: Multiple Choice
MCQ Answer Key
- B
- B
- A
- A
- C
- D
- C
- D
- A
- D
- A
- B
- C
- B
- D
- B
- A
- B
- C
- B
Question 1
A strong acid is one that completely ionises in solutions. Since the solution contains only H+ and A- ions, this suggests that the acid is fully ionised (HA(aq) → H+(aq) + A-(aq)) and therefore must be a strong acid.
Since there are very few acid molecules in the solution, this suggests the solution is dilute and not concentrated.
Therefore, option B is the correct answer.
Question 2
The reaction illustrates the conversion of butan-2-ol (a secondary alcohol) into butan-2-one (a ketone). This is performed via an oxidation reaction.
Therefore, option B is the correct answer.
Question 3
1,2-dibromopentane is the name of an alkane with a 5-carbon chain that has a bromine substituent on carbon 1 and 2.
This corresponds to option A.
Question 4
The addition of I– to the solution containing Pb2+ ions will result in a yellow precipitate forming: Pb2+(aq) + 2I–(aq) → PbI2(s). Adding I– to the solution containing Na+ will not produce a precipitate. This allows the two solutions to be identified. Therefore, option A is the correct answer.
The addition of NH4+, NO3–, and CH3COO– to both solutions will not result in any visible reaction, as no precipitate forms with any of the cations. Therefore, the addition of NH4+, NO3- and CH3COO- cannot be used to distinguish between the two solutions.
Question 5
Since there is initially some Cl2, the concentration of Cl2 is initially non-zero. This eliminates options B and D.
The concentration of Cl2 will decrease until it reaches a constant non-zero value when dynamic equilibrium is established.
Therefore, option C is the correct graph.
Question 6
HCl is a strong acid: HCl(aq) + H2O(l) → H3O+(aq) + Cl-(aq)
∴ [H3O+] = 0.25 molL-1 (1:1 stoichiometric ratio)
pH = -log100.25 = 0.60
Question 7
Since the two complexes are different in colour, UV-visible spectrophotometry is most suitable for distinguishing between the two complexes as they will absorb a different peak wavelengths in the visible region of the electromagnetic spectrum.
Therefore, option C is the correct answer.
Question 8
The molecular ion peaks of X and Y correspond to the molar mass of X and Y, respectively.
By the Law of Conservation of Mass: MM(X) + MM(unknown substance) = MM(Y)
∴ MM(unknown substance) = MM(Y) - MM(X) = 92 - 72 = 20 gmol-1
20gmol-1 ≈ MM(HF)
Therefore, option D is the correct answer.
Question 9
As saponin has both water-soluble and fat-soluble components, it will be able to act as a cleaning agent, similarly to soaps. This is because the fat-soluble component will interact with any grease, while the water-soluble component will interact with water molecules. Upon agitation, micelles will form around the grease, producing an emulsion which can be washed away, leaving the surface clean.
Therefore, the answer is A.
Question 10
Butanoic acid is a covalent molecule, so it can exhibit dispersion forces. It is also polar due to the presence of a carboxyl group, allowing it to form dipole-dipole interactions. Since the acidic proton is a hydrogen bond donor, while the lone pairs of electrons on the oxygen atoms are hydrogen bond acceptors, butanoic acid can also form hydrogen bonds.
Therefore, the correct answer is D.
Covalent bonding is an intramolecular force. It is not present between molecules of butanoic acid, but instead it is present within butanoic acid, holding the atoms together.
Question 11
A Brønsted-Lowry acid is a proton donor, while a Brønsted-Lowry base is a proton acceptor. X is propanoate, the weak conjugate base of propanoic acid and therefore can accept a proton, acting as a Brønsted-Lowry base. Y is ethanamine, a weak base and therefore is also capable of accepting a proton and acts as a Brønsted-Lowry base.
Therefore, A is the correct answer.
Question 12
Keq = [products] / [reactants] = [Fe(NO3)3] / ([AgNO3]3[FeCl3])
Chemicals in a liquid or solid state are omitted from the equilibrium expression, so AgCl(s) is not included.
Therefore, option B is the correct answer.
Question 13
Combustion of octane above 100°C: 2C8H18(l) + 25O2(g) → 16CO2(g) + 18H2O(g)
Note that water is gaseous as the reaction is occurring above 100°C.
Combustion is an exothermic reaction. Hence, the reaction has a negative enthalpy change,i.e. ∆H < 0. Since there are significantly more gaseous molecules on the products side than the reactants side, entropy increases in this process, i.e. ∆S > 0.
Therefore, option C is the correct answer.
Question 14
Experiment 1: Keq = [H2][I2] / [HI]2 = 0.012 / 0.042
Experiment 2: Keq = [H2][I2] / [HI]2 = 0.022 / 0.042
By comparing the expressions for Keq, Keq(experiment 2) > Keq(experiment 1). This eliminates options C and D.
Since Keq(experiment 2) is larger, this implies that the system shifts to the right when the temperature is changed from experiment 1. Since the forward reaction is endothermic, the temperature at which experiment 2 is performed must be higher than experiment 1 so that the equilibrium will shift to the right by Le Chatelier’s Principle to decrease temperature.
Therefore, option B is the correct answer.
Question 15
When prop-2-en-1-ol reacts with hydrogen gas, it undergoes hydrogenation, an addition reaction, to produce propan-1-ol.
Upon oxidation, since propan-1-ol is a primary alcohol, it will oxidise into propanoic acid. The identity of propanoic acid is further confirmed, as it effervesced upon the addition of sodium carbonate due to the production of carbon dioxide.
When propanoic acid is reacted under reflux with metanol and concentrated sulfuric acid, it undergoes an esterification reaction to form an ester, i.e. methyl propanoate.
Hence, X is propan-1-ol, Y is propanoic acid, and Z is methyl propanoate, and their structures are correctly illustrated by option D.
Question 16
These are two ways of approaching this question.
Method 1
MM(3-hydroxypropanoic acid) = 3 x 16.00 + 3 x 12.01 + 6 x 1.008 = 90.078 gmol-1
3-hydroxypropanoic acid undergoes homocondensation polymerisation. Therefore, when n monomer units react, (n-1) water molecules are produced.
∴ MM(strand) = MM(monomers)used - MM(H2O)condensed
= 1000 x 90.078 - 999 x (2 x 1.008 + 16.00) = 72080 gmol-1
Method 2
Repeating unit: –OCH2CH2CO–
MM(repeating unit) = 2 x 16 + 3 x 12.01 + 4 x 1.008 = 72.062 gmol-1
MM(strand) = 1000 x MM(repeating unit) + MM(H2O) = 72.062 x 1000 + (2 x 1.008 + 16) = 72080 gmol1
Here, the molar mass of water was added once in the expression to account for the H and OH on either end of the chain, which did not condense.
Question 17
The distinct chemical environments for each compound are provided below:
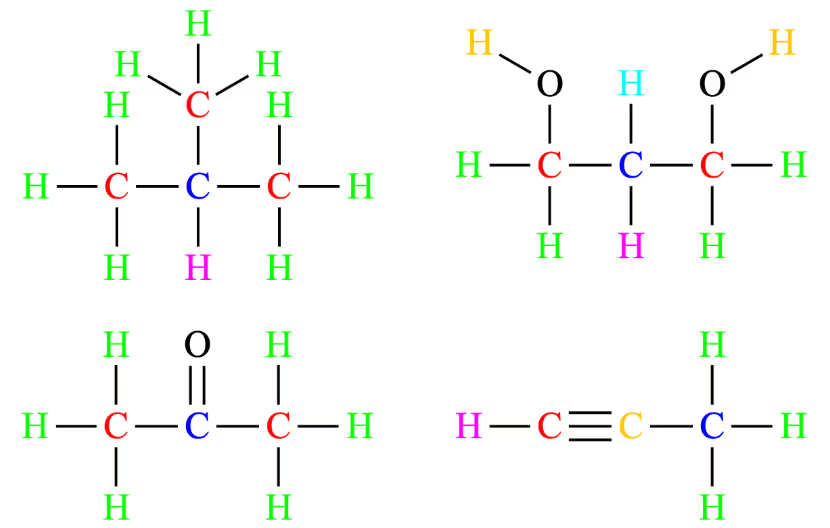
As can be seen, the only option where the number of carbon chemical environments equals the number of proton chemical environments is option A.
Question 18
This question is describing a modified version of Mohr's method.
When the CrO42–(aq) indicator is added to Ag+(aq) ions in the solution of unknown concentration, a precipitation reaction occurs immediately. This precipitates out the yellow CrO4–(aq) ions into the red Ag2CrO4(s). Hence, the solution is initially red.
CrO42–(aq) (yellow) + 2Ag+(aq) → Ag2CrO4(s) (red)
As sodium chloride is then added to the solution, the chloride ions precipitate out with the Ag+(aq) ions.
Cl–(aq) + Ag+(aq) → AgCl(s)
For the CrO42–(aq) ions to adequately act as an indicator, it must change in colour once all of the Cl–(aq) ions above are consumed. Thus, AgCl(s) must precipitate our preferentially, and is thus the less soluble salt.
As Cl–(aq) ions are continued to be added to the solution, Ag+(aq) ions which were originally part of the red Ag2CrO4(s) precipitate dissolve back in to precipitate out with AgCl(s) instead, the less soluble salt.
This results in the CrO42–(aq) ions being freed up in the process, and the solution turning yellow.
Thus, option A is the correct answer, which indicates that the solution changes in colour from red to yellow, and matches Ag2Cr2O4 as the more soluble salt.
Marker’s Notes
- This was a confusing question, as the term “concentration of [...] is determined by titrating it with [...]” doesn’t sufficiently indicate which solution is present in the flask to begin. In this case, the HSC paper intended for the phrase to indicate that the conical flask initially contains the silver ions.
- In the opinion of the tutors at Cognito, we believe option B is also an adequate answer, given this ambiguity in the phrasing.
Question 19
These are two ways of approaching this question.
Method 1 (More methodological)
The following reaction takes place and occurs to completion, as HCl(aq) is a strong acid:
CH3COO–(aq) + HCl(aq) → CH3COOH(aq) + Cl–(aq)
n(CH3COO–) = 0.1 mol
n(HCl) = 0.500 L x 0.1 molL-1 = 0.05 mol
Since the two species react in a 1:1 ratio, HCl(aq) is the limiting reagent.
After the reaction,
n(CH3COO–)after neutralisation = n(CH3COO–)initial - n(CH3COO–)reacted
= n(CH3COO–)initial - n(HCl)added
= 0.1 mol - 0.05 mol
= 0.05 mol
n(CH3COOH)after neutralisation = n(HCl)added
= 0.05 mol
The following equilibrium is then established:
CH3COOH(aq) + H2O(l) ⇌ CH3COO–(aq) + H3O+(aq)
Hence, constructing an ICE table:
| [CH3COOH] (molL-1) | [CH3COO–] (molL-1) | [H3O+] (molL-1) | |
|---|---|---|---|
| Initial | 0.05 mol / 0.5 L = 0.1 | 0.05 mol / 0.5 L = 0.1 | 0 |
| Change | - 10–4.8 | + 10–4.8 | + 10–4.8 |
| Equilibrium | 0.1 - 10–4.8 | 0.1 + 10–4.8 | 10–4.8 |
Hence, Ka = [CH3COO–][OH–] / [CH3COOH]
= (0.1 + 10–4.8)(10–4.8) / (0.1 – 10–4.8)
= 1.585395 x 10-5
Then, considering the dilution:
| [CH3COOH] (molL-1) | [CH3COO–] (molL-1) | [H3O+] (molL-1) | |
|---|---|---|---|
| Initial | 0.1 x (500 mL / 1000 mL) = 0.05 | 0.1 x (500 mL / 1000 mL) = 0.05 | 0 |
| Change | - x | + x | + x |
| Equilibrium | 0.05 – x | 0.5 + x | x |
Substituting and solving, we have:
Keq = x(0.05 + x) / (0.05 – x) = 1.585395 x 10–5
As Keq is small, x is small and hence 0.5 + x ≈ 0.5 – x ≈ 0.5 (small change assumption)
⇒ x = 1.585395 x 10–5
Thus, pH = -log10[H3O+] = -log10(1.585395 x 10-5) = 4.79986252 = 4.8 (1 d.p.)
Method 2 (Faster)
Since exactly half of the CH3COO–(aq) are neutralised by the HCl(aq) to CH3COOH(aq), this represents a buffer solution / half-equivalence point, where:
[CH3COO–]initial = [CH3COOH]initial
Hence, making the small change assumption that [CH3COO–]initial ≈ [CH3COO–]eq and [CH3COOH]initial ≈ [CH3COOH]eq:
Ka = [CH3COO–][H3O+] / [CH3COOH] = [H3O+] = 10-4.8
In other words, [H3O+] is equal to the Ka and independent of the actual concentration of the acid or the volume of water present.
Thus, the pH does not change and remains at 4.8, which corresponds to option C.
Question 20
[Ag+]diluted = 0.11 x 10-6 molL-1 (by interpolation)
Since it is given that [Ag+]saturated / [Ag+]diluted = 2000, it follows that:
[Ag+]saturated = 2000 x [Ag+]diluted
= 2000 x (0.11 x 10-6 molL-1)
= 2.2 x 10-4 molL-1
Consider the actual dissolution of silver oxalate:
Ag2C2O4(s) ⇌ 2Ag+(aq) + C2O42-(aq)
Since Ag+(aq) and C2O42-(aq) are produced in 2:1 ratio,
[C2O42-]saturated = ½ x [Ag+]saturated
= ½ x (2.2 x 10-4 molL-1)
= 1.1 x 10-4 molL-1
Hence, Ksp = [Ag+]2[C2O42-]
= (2.2 x 10-4)2(1.1 x 10-4)
= 5.3 x 10-12
Section II: Written Responses
Question 21
Exemplar Answer
| Reaction condition X | IUPAC name of organic product |
|---|---|
| UV light | 2-bromobutane |
Question 22
Exemplar Answer
Qualitative analysis
Qualitative analysis is used to identify chemical species in the water, which may be environmental contaminants such as NO3-(aq), which can cause eutrophication or Pb2+(aq) ions, which are toxic and can bioaccumulate and biomagnify.
Quantitative analysis
Quantitative analysis allows the concentration of each chemical species to be calculated. This allows chemists to monitor the concentration of species to ensure they are within safe limits and implement management strategies when they trend above the safe limits.
Question 23
Exemplar Answer
- The mass of the watch glass and filter paper have not been measured separately from the BaSO4 precipitate. This results in a systematic error, where the mass of the washglass and filter paper inflates the mass of the precipitate and reduces the accuracy of the % w/w calculated. These pieces of equipment should be weighed separately and subtracted from the mass measured in step 7.
- The precipitate should be weighed in multiple 20-minute intervals throughout the drying process until a constant mass is obtained. This would ensure that all of the moisture is removed, which could otherwise inflate the mass of the precipitate and act as a systematic error that reduces accuracy.
Answers Could Include
- A higher concentration of BaCl2(aq) could be added to ensure that the sulfate ions are precipitated out completely.
- The drying of the precipitate could be performed in a more controlled environment in the lab, such as a dessicator or a drying oven. This would minimise the likelihood of impurities such as pollens or dust settling on the watch glass, or some of the precipitate being lifted away by the wind. These random errors would otherwise reduce the reliability of the % w/w measured.
- Any solids should be filtered out before the BaCl2(aq) solution is added, as the fertiliser sample may contain many insoluble components. If these insoluble components are left in the solution, it will also contribute to the measured mass of BaSO4(s) precipitate, resulting in a systematic error.
Question 24(a)
Exemplar Answer
Structural formula
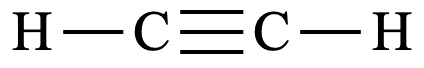
Shape of molecule: Linear
Question 24(b)
Exemplar Answer
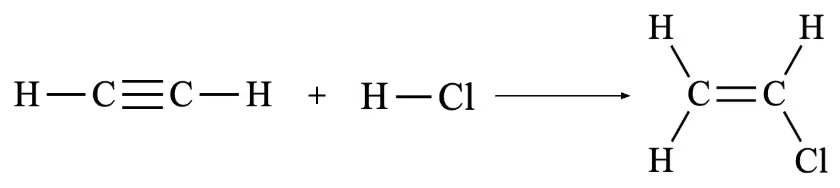
n(C2H2) = m(C2H2) / MM(C2H2)
= 65 g / 26.04 gmol-1
= 2.49654325 mol
Since n(C2H2)used = n(C2H3Cl)produced = 1:1
n(C2H3Cl)produced = n(C2H2)used = 2.49654325 mol
Hence, m(C2H3Cl)produced = n(C2H3Cl)produced x MM(C2H3Cl)
= 2.49654325 mol x 62.50 gmol-1
= 156.033953 g
= 156 g (3 s.f.)
Question 25
Exemplar Answer
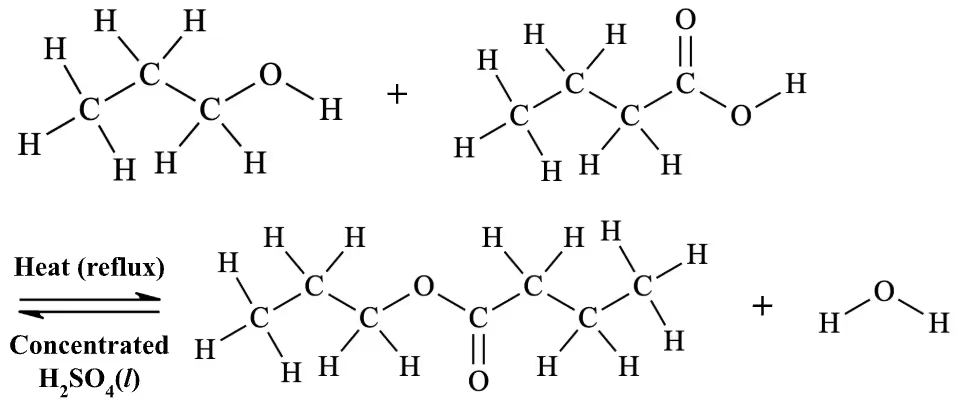
Propan-1-ol and butanoic acid react in 1:1 ratio.
Since n(propan-1-ol) = 0.267 mol < n(butanoic acid) = 0.298 mol, propan-1-ol is the limiting reagent.
Since n(propan-1-ol)used:n(propyl butanoate)theoretically produced = 1:1,
n(propyl butanoate)theoretically produced = n(propan-1-ol)used
= 0.267 mol
m(propyl butanoate)theoretically produced = 0.267 mol x 130.2 gmol-1 = 34.7634 g
Now, calculating the mass actually produced:
m(propyl butanoate)actually produced = 12.2 mL x 0.873 gmL-1 = 10.6506 g
Hence, % yield = (m(propyl butanoate)actually produced / m(propyl butanoate)theoretical produced) x 100%
= (10.6506 g / 34.7634 g) x 100%
= 30.6373945%
= 30.6% (3 s.f.)
Question 26(a)
Exemplar Answer
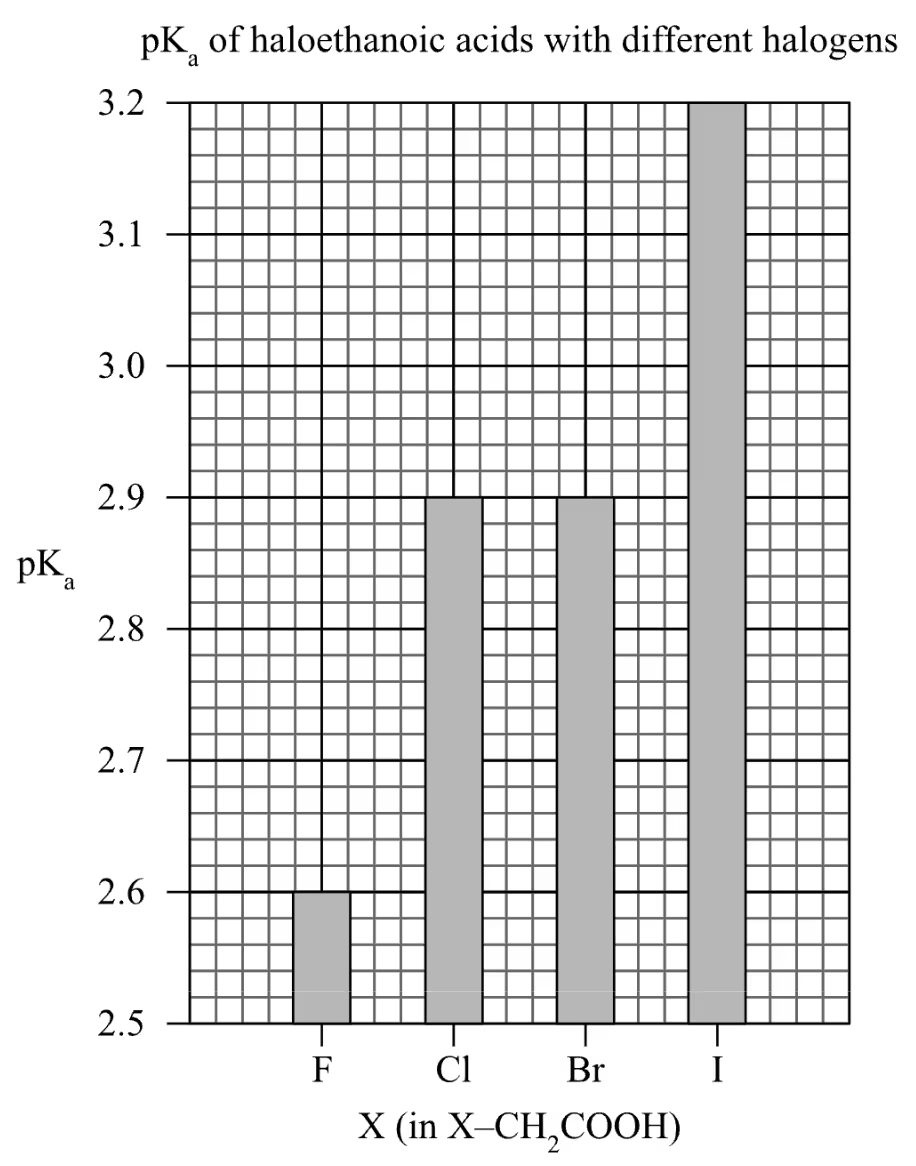
Marker’s Notes
- As the horizontal axis is discrete in nature, a column graph is preferred.
Question 26(b)
Exemplar Answer
As halogens are taken from further down in Group 17, the pKa of the haloethanoic acid increases. Since Ka = 10-pKa, the increase in pKa corresponds to a decrease in Ka. As Ka = [X–CH2COO–][H3O+] / [X–CH2COOH] decreases, the concentration of the ionised acid at equilibrium decreases.
Since the greater the degree of ionisation of an acid, the stronger the acid is, the strength of the acid decreases as the halogen in the haloethanoic acid is found further down Group 17.
Question 27(a)
Exemplar Answer
The combustion of petrol in engines releases greenhouse gases such as CO2(g) and / or CO(g): C7H16(l) + 11O2(g) → 7CO2(g) + 8H2O(l).
These greenhouse gases contribute to the greenhouse effect, which prevents sunlight from escaping into space after being reflected by the Earth. This results in a rise in the climate temperature (global warming).
This results in increasing frequency and severity of bushfires, as well as the melting of ice caps and a consequent rise in sea level. In either case, natural habitat is lost, which is a detrimental implication for the environment.
Question 27(b)
Exemplar Answer
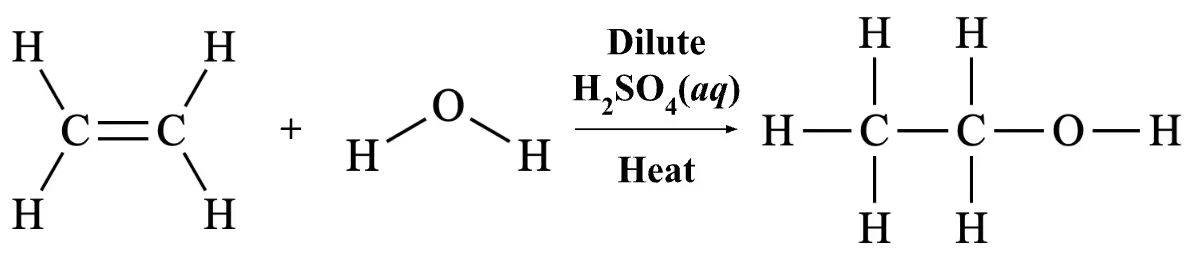
Question 27(c)
Exemplar Answer
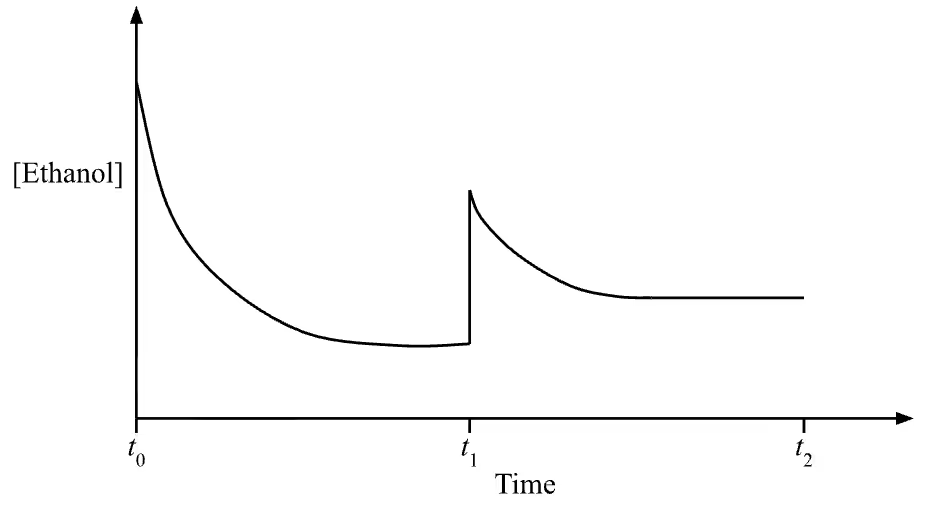
Question 28(a)
Exemplar Answer
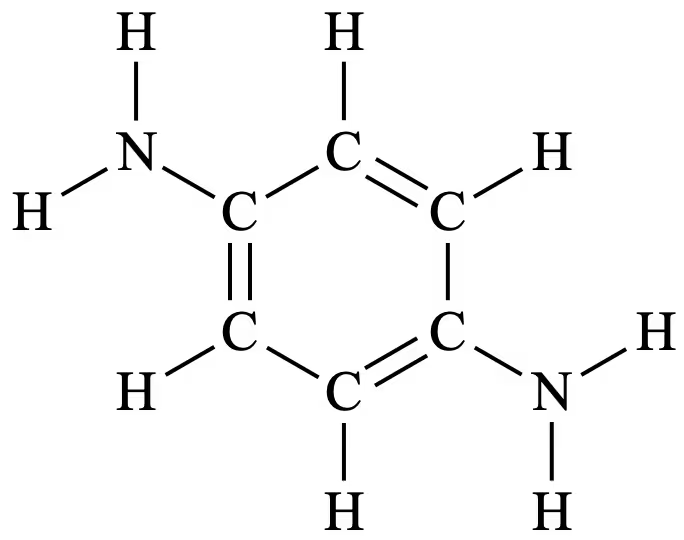
Question 28(b)
Exemplar Answer
The stronger the intermolecular forces, the greater the amount of energy required to separate the polymer chains, resulting in a greater difficulty in pulling them apart, as well as an increasing melting point.
Kevlar is a condensation polymer featuring amide linkages. Thus, it possesses a polar amide functional group throughout the polymer, resulting in strong polar hydrogen bonding between Kevlar molecules, as well as dispersion forces.
Meanwhile, Polystyrene is an addition polymer without polar functional groups. Thus, it is non-polar and can only form weak dispersion forces between each polymer molecule. In addition, its large benzene ring side chain prevents efficient stacking and creates chain chain-stiffening effect, which increases the brittleness of the polymer.
Hence, Kevlar has stronger intermolecular forces than Polystyrene, as well as being less brittle. Thus, Kevlar is harder to pull apart than polystyrene, and it possesses a higher melting point.
Marker's Notes
Since the question specifies physical properties in plural, more than one physical property should be discussed.
Question 29
Exemplar Answer
Since the system is initially at equilibrium, Q = [N2O4] / [NO2]2= Keq. When argon gas is added to the system, as the volume is fixed, the concentrations of NO2 and N2O4 do not change. Therefore, Q = Keq after the addition of argon gas, and the system does not shift in either direction, remaining at equilibrium.
As there are no macroscopic changes to a system at dynamic equilibrium, the temperature will remain constant.
Question 30(a)
Exemplar Answer
As phosgene is a toxic gas, it should be handled in a fume cupboard to prevent its diffusion and inhalation. Personal protective equipment such as gas marks may be worn by all staff to further prevent inhalation.
Question 30(b)
Exemplar Answer
Excess carbon monoxide
When an excess of carbon monoxide is injected into the system. By Le Chatelier's Principle, the position of equilibrium will shift right to decrease the carbon monoxide concentration. Consequently, the yield of phosgene will increase. This increases the profitability of the process, and is thus desirable.
Use of a catalyst
The use of a catalyst provides an alternate reaction pathway with a lower activation energy. This results in a greater success rate of collisions, as a greater proportion of particles can collide with sufficient energy above the activation energy barrier. Therefore, the rate at which phosgene is synthesised will increase, increasing the yield of phosgene. This increase in the rate of production ultimately increases the profitability of the process.
Question 31(a)
Exemplar Answer
By Gay-Lussac’s Law of Combining Volumes, the volume ratio of gases in a reaction matches the stoichiometric ratio.
N2H4(g) + 3O2(g) → 2NO2(g) + 2H2O(g)
Using N2H4 as the molecular formula for hydrazine gives a stoichiometric ratio of n(hydrazine):n(O2):n(NO2):n(H2O) = 1:3:2:2, which matches the volume ratio provided of V(hydrazine):V(O2):V(NO2):V(H2O) = 1 L : 3 L : 2 L : 2 L.
Question 31(b)
Exemplar Answer
Ka(N2H5+) = Kw / Kb(N2H4) = 10-14 / (1.7 x 10-6) = 5.88235 x 10-9
N2H5+(aq) + H2O(l) ⇌ N2H4(aq) + H3O+(aq)
Let x be [H3O+]eq:
| [N2H5+] (molL-1) | [N2H4] (molL-1) | [H3O+] (molL-1) | |
|---|---|---|---|
| Initial | 0.20 | 0 | 0 |
| Change | - x | + x | + x |
| Equilibrium | 0.20 – x | x | x |
Ka = [H3O+] [N2H4] / [N2H5+]
⇒ x2 / (0.2 - x) = 5.88235 x 10-9
As Ka is small, x is small and hence 0.20 - x ≈ 0.20 (small change assumption)
⇒ x2 / 0.2 = 5.88235 x 10-9
⇒ x = 3.429971 x 10-5 = [H3O+]
pH = –log10[H3O+]
= –log10(3.429971 x 10-5)
= 4.46 (2 d.p.)
Question 32
Exemplar Answer
As all sodium and nitrate salts are soluble, the only precipitates that can form are Mg(OH)2(s) and MgCO3(s).
As the Ksp of Mg(OH)2 = 5.61 x 10-12 is significantly smaller than the Ksp of MgCO3 = 6.82 x 10-6, Mg(OH)2 will precipitate out first.
[Mg2+] = 0.006 mol / 1 L = 0.006 molL-1
[OH–] = 0.010 mol / 1 L = 0.01 molL-1
Mg(OH)2(s) ⇌ Mg2+(aq) + 2OH–(aq)
Qsp = [Mg2+][OH–]2 = 0.006 x 0.012 = 6 x 10-7
Ksp(Mg(OH)2) = 5.61 x 10-12
As Qsp > Ksp, Mg(OH)2 will form a precipitate.
Assuming the precipitation reaction goes to completion (as Ksp is very small):
Mg2+(aq) + 2OH–(aq) → Mg(OH)2(s)
n(Mg2+) = 0.006 mol
n(OH–) = 0.01 mol
Since ½ x n(OH–) = 0.005 mol < n(Mg2+), OH– is the limiting reagent (1:2 stoichiometric ratio)
n(Mg2+)excess = 0.006 mol - (0.01 mol / 2) = 0.001 mol
[Mg2+]excess = 0.001 mol / 1 L = 0.001 molL-1
[CO32-] = 0.002 mol / 1 L = 0.002 molL-1
MgCO3(s) ⇌ Mg2+(aq) + CO32-(aq)
Qsp = [Mg2+][CO32-] = 0.001 x 0.002 = 2 x 10-6
Ksp(MgCO3) = 6.82 x 10-6
As Qsp < Ksp, MgCO3 will not precipitate.
Hence, the only precipitate that forms is Mg(OH)2.
Marker’s Notes
The solution above reasonably assumes that the precipitation of Mg(OH)2 goes effectively to completion. Without such an option, the question can be approached as follows.
Mg(OH)2(s) ⇌ Mg2+(aq) + 2OH–(aq)
MgCO3(s) ⇌ Mg2+(aq) + CO32–(aq)
Let the amount of Mg(OH)2 that precipitates in molL-1 be x. Then:
| [Mg2+] (molL-1) | [OH–] (molL-1) | |
|---|---|---|
| Initial | 0.006 | 0.010 |
| Change | - x | - 2x |
| Equilibrium | 0.006 - x | 0.010 - 2x |
Then, at the point where Mg(OH)2 ceases to precipitate:
| [Mg2+] (molL-1) | [OH–] (molL-1) | |
|---|---|---|
| Initial | 0.006 | 0.010 |
| Change | - (0.005 - 0.5z) | - (0.010 - z) |
| Equilibrium | 0.001 + 0.5z | z |
Qsp = Ksp
⇒ [Mg2+][OH–]2 = Ksp
⇒ (0.001 + 0.5z)(z)2 = 5.61 x 10-12
Since Ksp is small, z is small. Thus, applying the small change assumption, 0.001 + 0.5z ≈ 0.001.
(0.001)(z)2 = 5.61 x 10-12
⇒ z2 = 5.61 x 10-9
⇒ z = 7.49 x 10-5
Hence, [Mg2+]saturation of Mg(OH)2 = 0.001 + 0.5z = 0.001 + 0.5 x 7.49 x 10-9 = 0.00103745 molL-1
At this point, Qsp of MgCO3 = [Mg2+][CO32-] = (0.00103745)(0.002) = 0.00000207 (< Ksp of MgCO3 = 6.82 x 10-6)
Hence, MgCO3(s) does not precipitate out, and the only precipitate that forms is Mg(OH)2.
Question 33
Exemplar Answer
V(KOH) = (7.20 + 7.10 + 7.15) / 3 = 7.15 mL = 0.00715 L (ignoring 7.80 mL as an outlier)
n(KOH) = 0.1 molL-1 x 0.00715 L
= 0.000715 mol
HCl(aq) + KOH(aq) → KCl(aq) + H2O(l)
n(HCl)titrated = 0.000715 mol (based on 1:1 stoichiometric ratio)
n(HCl)excess = 0.000715 mol x (100.0 mL / 20 mL)
= 0.003575 mol
n(HCl)initial = 0.1 L x 0.550 molL-1
= 0.0550 mol
n(HCl)reacted with CaCO3 = n(HCl)initial - n(HCl)excess
= 0.0550 mol - 0.003575 mol
= 0.051425 mol
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
n(CaCO3) = 0.051425 / 2 (based on 1:2 stoichiometric ratio)
= 0.0257125 mol
m(CaCO3) = 0.0257125 mol x (40.08 + 12.01 + 3 x 16.00) gmol-1
= 2.573564125 g
%w/w(CaCO3) = (2.573564125 / 3) x 100%
= 85.78547%
≈ 85.5%
Therefore, Brand X was used.
Question 34(a)
Exemplar Answer
Method 1 (Half-equivalence point)
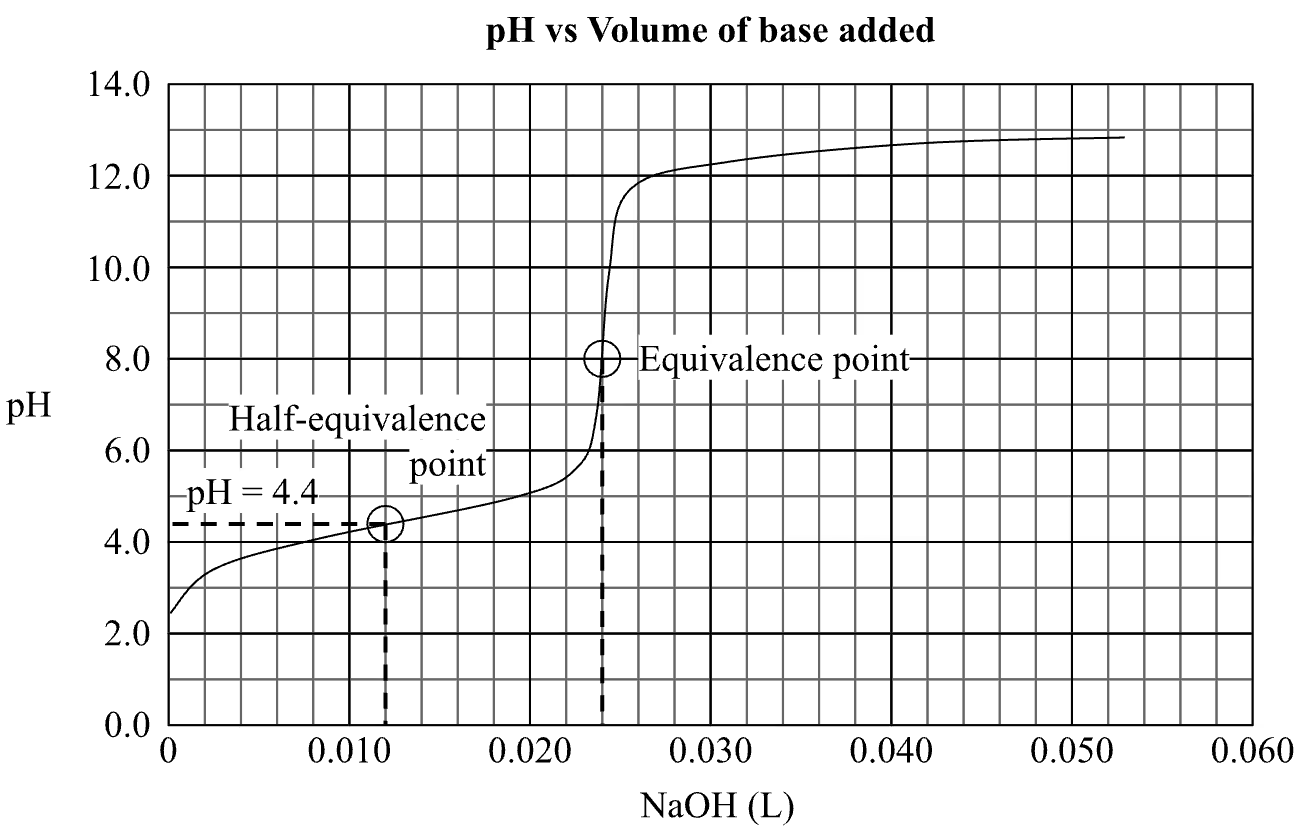
The equivalence point occurs at the point where 0.024 L of NaOH has been added.
The half-equivalence point must thus occur at the point where exactly half of that amount of NaOH has been added, at V(NaOH) = 0.024 / 2 = 0.012 L, which corresponds to a pH of 4.4.
At the half-equivalence point, [HX] = [X–], so Ka = [X–][H+] / [HX] = [H+], and pKa = pH = 4.4.
Hence, Ka = 10-pKa = 10–4.4 ≈ 4.0 x 10-5 (2 s.f.)
Method 2 (Initial pH)
V(NaOH)required = 0.024 L
n(NaOH)required = 0.024 L x 0.10 molL-1 = 0.0024 mol
HX(aq)+ NaOH(aq) → NaX(aq) + H2O(l)
Since HX(aq) and NaOH(aq) react in 1:1 ratio,
n(HX) = n(NaOH) = 0.0024 mol
Hence, [HX]initial = 0.0024 mol / 0.010 L = 0.24 molL-1
Based on the initial pH on the titration curve, [H+]eq = 10-2.5 molL-1
HX(aq) ⇌ H+(aq) + X–(aq)
| [HX] (molL-1) | [H+] (molL-1) | [X-] (molL-1) | |
|---|---|---|---|
| Initial | 0.24 | 0 | 0 |
| Change | - 10-2.5 | + 10-2.5 | + 10-2.5 |
| Equilibrium | 0.24 – 10-2.5 | 10-2.5 | 10-2.5 |
Thus, Ka = [X–][H+] / [HX]
= (10-2.5)2 / (0.24 - 10-2.5)
= 0.000042223
≈ 4.2 x 10-5 (2 s.f.)
Question 34(b)
Exemplar Answer
At the equivalence point, when 0.024 L of NaOH is added, all of the acid (HX) is neutralised:
HX(aq) + NaOH(aq) → NaX(aq) + H2O(l)
As NaOH is further added after the equivalence point, it is diluted by the 0.010 L (acid) + 0.024 L (base) = 0.034 L of salt solution present. This results in the overall concentration of NaOH always being less than the 0.10 molL-1 NaOH solution added. Since pOH = -log10[OH-], the pOH of the solution will always be > 1. Further, since pH = 14 - pOH (at 25°C), the pH of the solution will always be less than 13 and never reach 13.
Question 35
Exemplar Answer
The reaction is exothermic in the forward direction, as the products have a lower enthalpy than the reactants.
Le Chatelier's Principle
Le Chatelier’s Principle states that when a system at dynamic equilibrium is disturbed the equilibrium will shift to minimise the disturbance until a new equilibrium is established.
When the solution is cooled from 80ºC to 0ºC, by Le Chatelier's Principle, the system will shift to increase the temperature by favouring the forward exothermic reaction, which releases heat. Therefore, the equilibrium shifts to the right and increases the concentration of [Co(H2O)6]2+, which is pink. Hence, the solution turns pink.
Collision theory
Collision theory states that for a chemical reaction to occur, chemicals must collide with sufficient energy (above the activation energy) with the correct molecular orientation.
When the solution is cooled to 0ºC, the collisional frequency of all particles decreases, and the proportion of particles that can overcome the activation energy barriers decreases, which results in the success rate of collisions also decreasing. Thus, both reaction rates decrease. However, as the reverse, endothermic reaction has a greater activation energy (Ea2) than that of the forward, exothermic reaction (Ea1), the reverse reaction experiences a greater decrease in the proportion of particles that can now overcome the activation energy barrier. Thus, the reverse reaction rate decreases to a greater extent, and the forward reaction is favoured, shifting the equilibrium to the right and increasing the concentration of [Co(H2O)6]2+, which turns the solution pink.
Question 36
Exemplar Answer
IR Spectrum
A very broad, strong trough will be observed in the wavenumber range of 2500 - 3000 cm-1 due to the presence of an acidic O–H bond in the carboxyl group. A sharp, strong trough will be observed in the wavenumber range of 1680 - 1750 cm-1 due to the presence of the C=O bond in the carboxyl group.
13C NMR
3 signals would be present on the 13C NMR spectrum, as the compound has 3 distinct carbon environments, as labelled below:
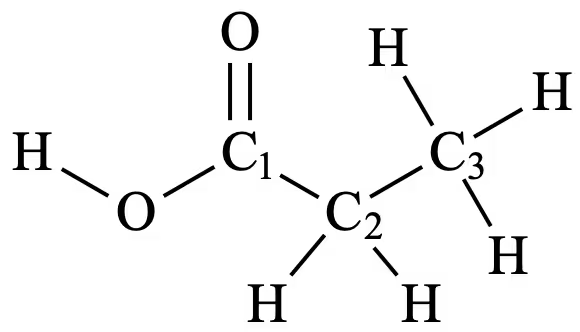
- C1 produces a signal between 160 – 185 ppm as it is the carbon in the carbonyl group of a carboxylic acid. This peak is significantly downfield due to the electronegative oxygen atoms deshielding C1 as they draw electron density away from it.
- C2 produces a signal from 20 – 50 ppm as it is the carbon atom adjacent to the carbonyl group.
- C3 produces a signal from 5 – 40 ppm, as it is not in proximity to any electronegative elements and produces a peak corresponding to C–C carbons. This signal will be more upfield than that produced by C2, as it is further away from the electronegative oxygen atom and hence is more shielded.
Proton NMR
3 signals would be present on the proton NMR spectrum as the compound has 3 distinct hydrogen environments, as labelled below:
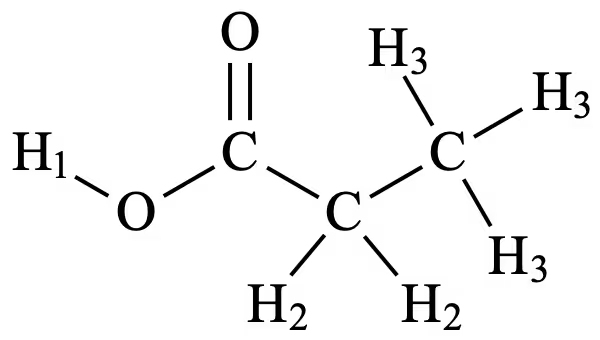
- H1 would produce the most downfield signal as it is directly bonded to an electronegative oxygen atom in the carboxyl group, resulting in the hydrogen atom being highly deshielded. As this hydrogen is directly bonded to oxygen, it produces a singlet in the spectrum.
- The two hydrogens in H2 would produce a signal from 2.1 – 4.5 ppm, as it corresponds to a CH2–CO– group on the carboxylic acid. The two hydrogens in H2 are neighboured by the 3 hydrogens in H3 and would hence produce a quartet by the n+1 rule of spin-spin coupling.
- The three hydrogens in H3 would produce a signal from 0.7 – 2.1 ppm as they correspond to –CH3 protons as seen on the data sheet. These three hydrogens are neighboured by the 2 hydrogens in H2 and would hence produce a triplet by the n+1 rule of spin-spin coupling.
The signal integral ratio of the three signals produced by H1, H2, and H3 environments would be 1:2:3, corresponding to the number of hydrogen atoms present in each environment.
Mass Spectroscopy
The molecular ion peak would be observed at 74 m/z, as propanoic acid has a molar mass of approximately 74 gmol-1. A smaller peak would likely appear at 75 m/z due to the presence of isotopes such as 13C.
Potential fragments that would produce peaks on the mass spectrum are summarised in the table below:
| Fragment | m/z ratio |
|---|---|
| COOH+ | 45 |
| CH3CH2CO+ | 57 |
| CH3CH2+ | 29 |
| CH3+ | 15 |
Marker’s Notes
The HSC has mistakenly termed mass spectrometry as mass spectroscopy in this question.
Question 37
Exemplar Answer
C5H10 matches the chemical formula of a 5-carbon alkene. It undergoes a hydration reaction (H+ / H2O) to produce compounds B and C. Thus, B and C must be alcohols.
As compound B does not react with acidified dichromate (H+ / Cr2O72-), this means it cannot oxidise and thus must be a tertiary alcohol.
As compound C does react with acidified dichromate, this means compound C can be oxidised, and as a result, must be a primary or secondary alcohol.
Compound D, the product of the oxidation of compound C, does not react with Na2CO3. This means compound D cannot be a carboxylic acid. As primary alcohols oxidise into carboxylic acids, compound C must therefore be a secondary alcohol, as compound D would be a ketone, which does not react with Na2CO3.
Thus, compound A is a 5-carbon alkene that hydrates to produce both secondary and tertiary alcohols. This means one of the carbon atoms in the C=C bond must be bonded to three other carbon atoms, while the other must be bonded to two.
Therefore, compound A is 2-methylbut-2-ene.
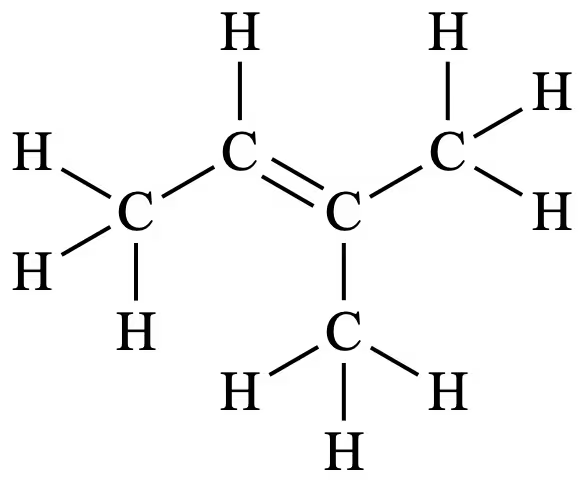
Marker’s Notes
- It should be noted that compound B is 2-methylbutan-2-ol, compound C is 3-methylbutan-2-ol, and compound D is 3-methylbutan-2-one.
- The name of compound A does not need to be provided to score full marks.
Marker’s Notes
The HSC has mistakenly termed mass spectrometry as mass spectroscopy in this question.
---
The small classes and exam-based approach at Cognito allows our talented, state-ranking tutors to personally help you get from where you're at to producing top-quality responses like these. Try out our 2-week obligation-free trial today and supercharge your HSC marks!